3D Printing Services
From concept to rapid prototyping to production and post-processing, we can help you achieve the full potential of additive manufacturing. Our experienced engineering team can help you select the right additive technology, material and process to meet your needs and accelerate your time to market. We offer full post-processing capabilities under one roof and can deliver additive parts as fast as next day.

Explore Fathom’s
Additive Manufacturing Solutions
Learn more about our 3D printing services and capabilities:
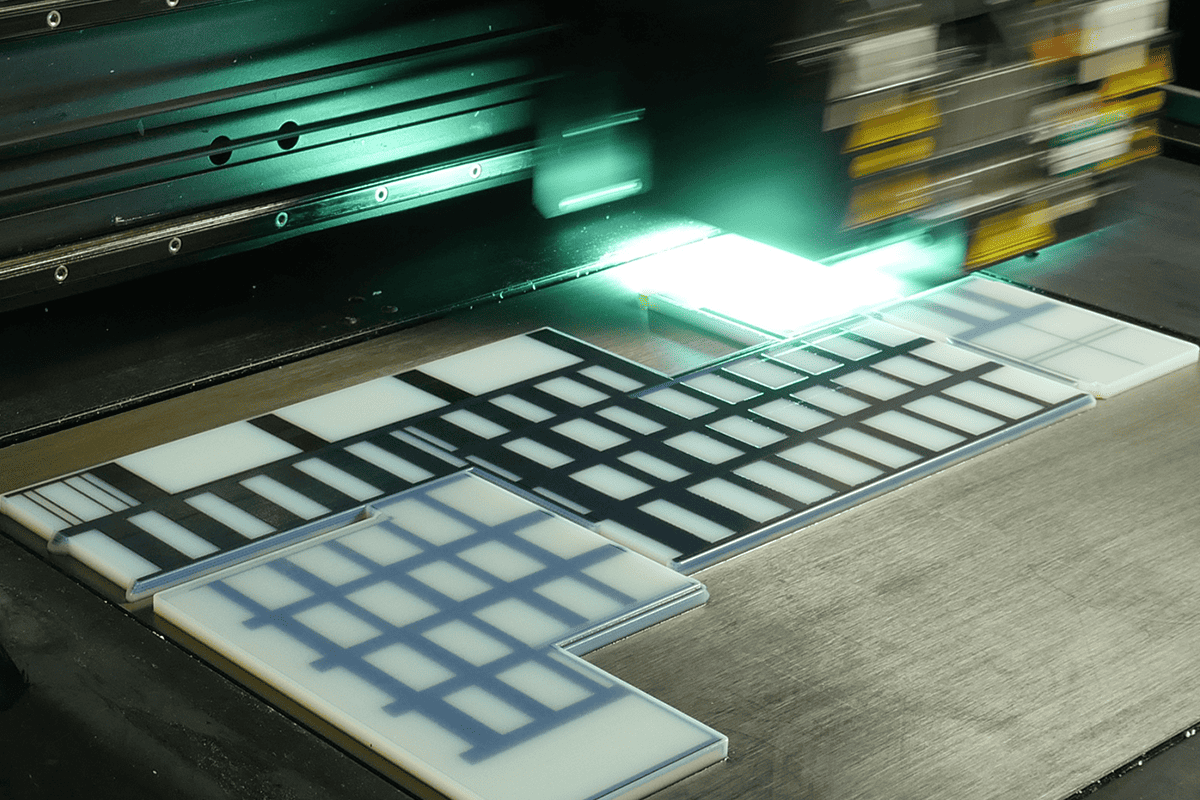
PolyJet
Do you need to create colorful demonstration models that explain concepts or validate a design? PolyJet enables the production of highly accurate parts with different durometers, from rigid to rubber-like consistencies, multi-color, transparent and translucent materials – all in a single build.
Fused Deposition Modeling (FDM)
FDM parts are made from a variety of thermoplastic materials and tend to be tougher and more robust than other additive technologies. That makes them ideal for demanding applications like jigs and work-holding fixtures.
Selective Laser Sintering (SLS)
Selective Laser Sintering (SLS) gives you the freedom to create intricate, high-quality parts with unmatched precision. SLS parts tend to be very durable with excellent surface characteristics.
STEP Technology
If you need tough, durable, high-precision prototype thermoplastic parts – fast – then you ought to investigate Selective Thermoplastic Electrophotographic Process (STEP). It’s a new technology available exclusively from Fathom that produces 3D-printed parts at injection molding speed – without tooling.
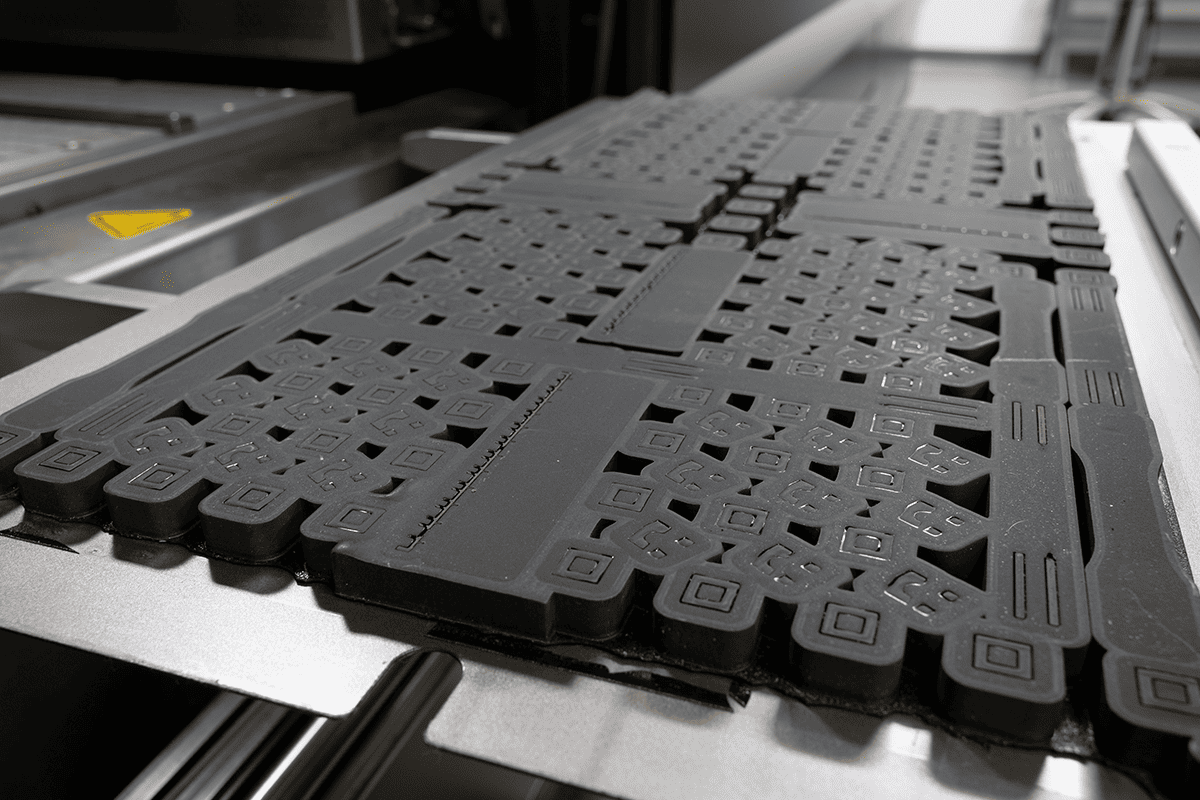
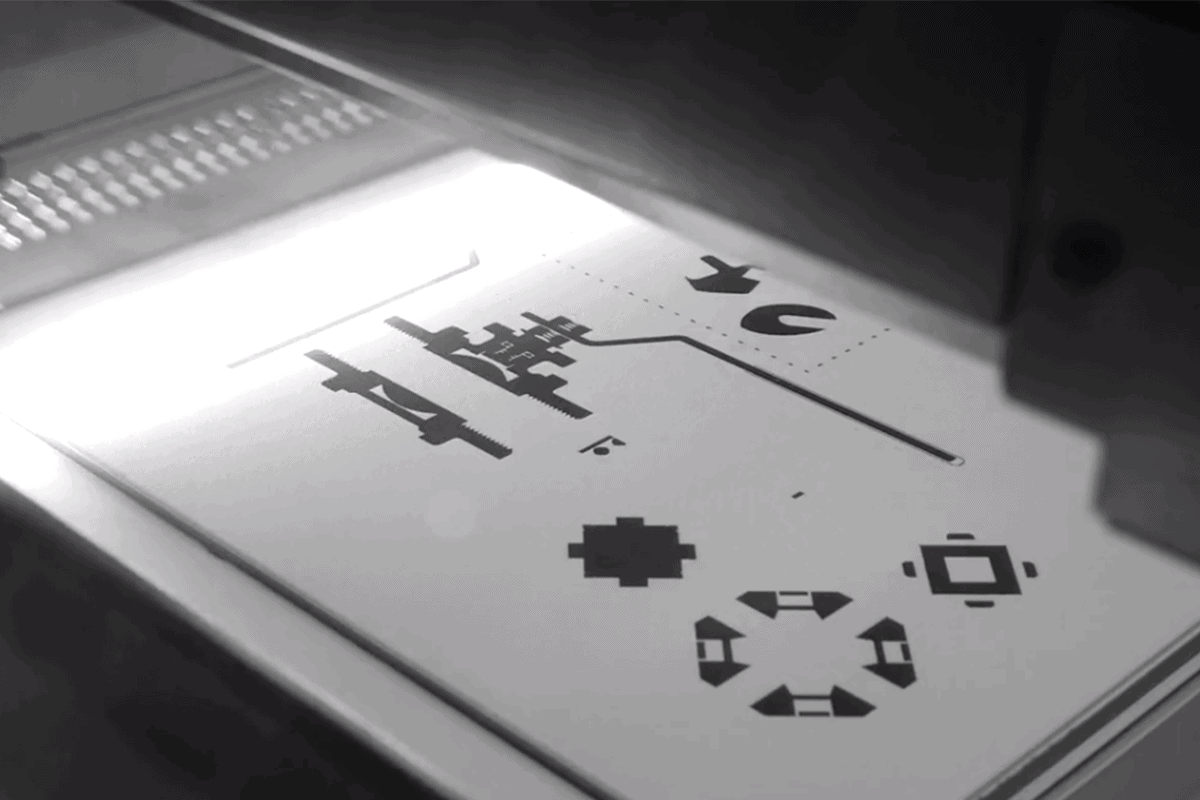
Multi Jet Fusion (MJF)
Do you need complex parts with highly accurate features and isotropic mechanical properties that can be produced quickly? MJF is an excellent choice for this common scenario. It can be used to produce functional prototypes as well as end-use parts.
Stereolithography (SLA)
If you’re looking for a fast, low-cost way to produce presentation models or test the fit and finish of prototype parts, SLA is an ideal solution. Designers rely on it to help refine and validate their ideas by creating affordable, high-quality physical models.
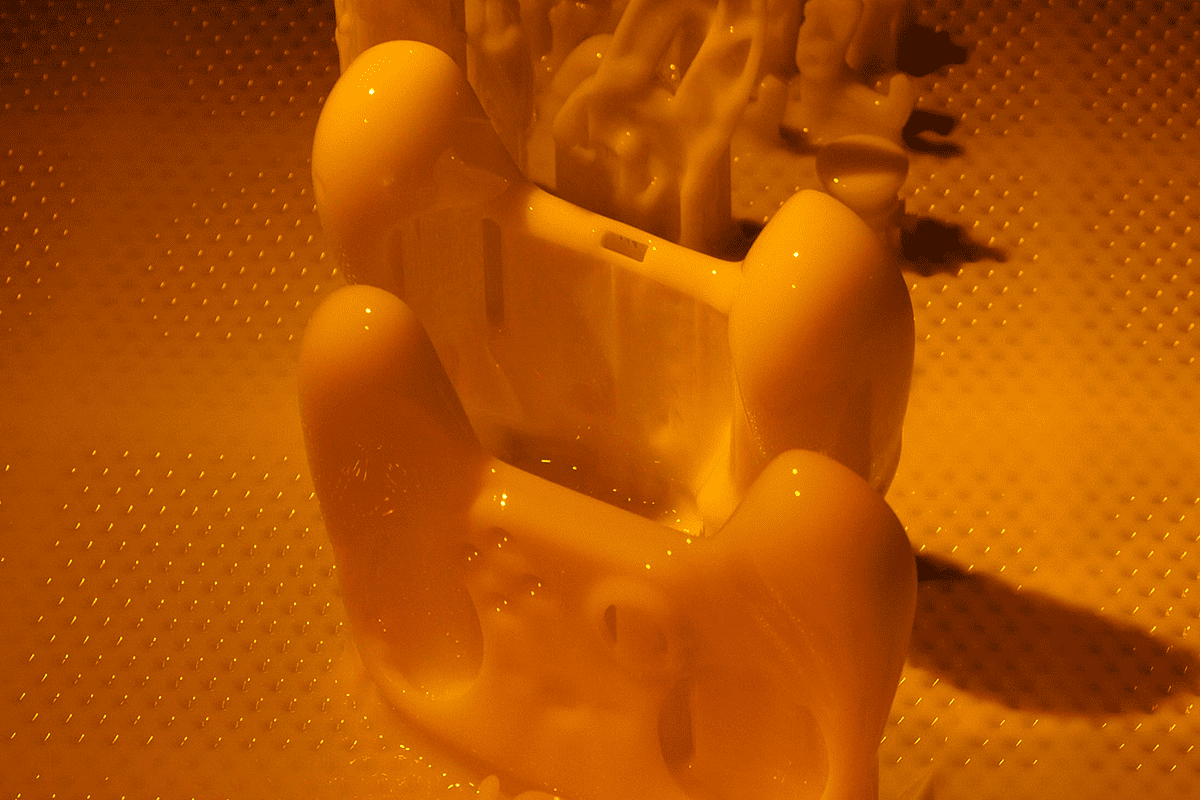
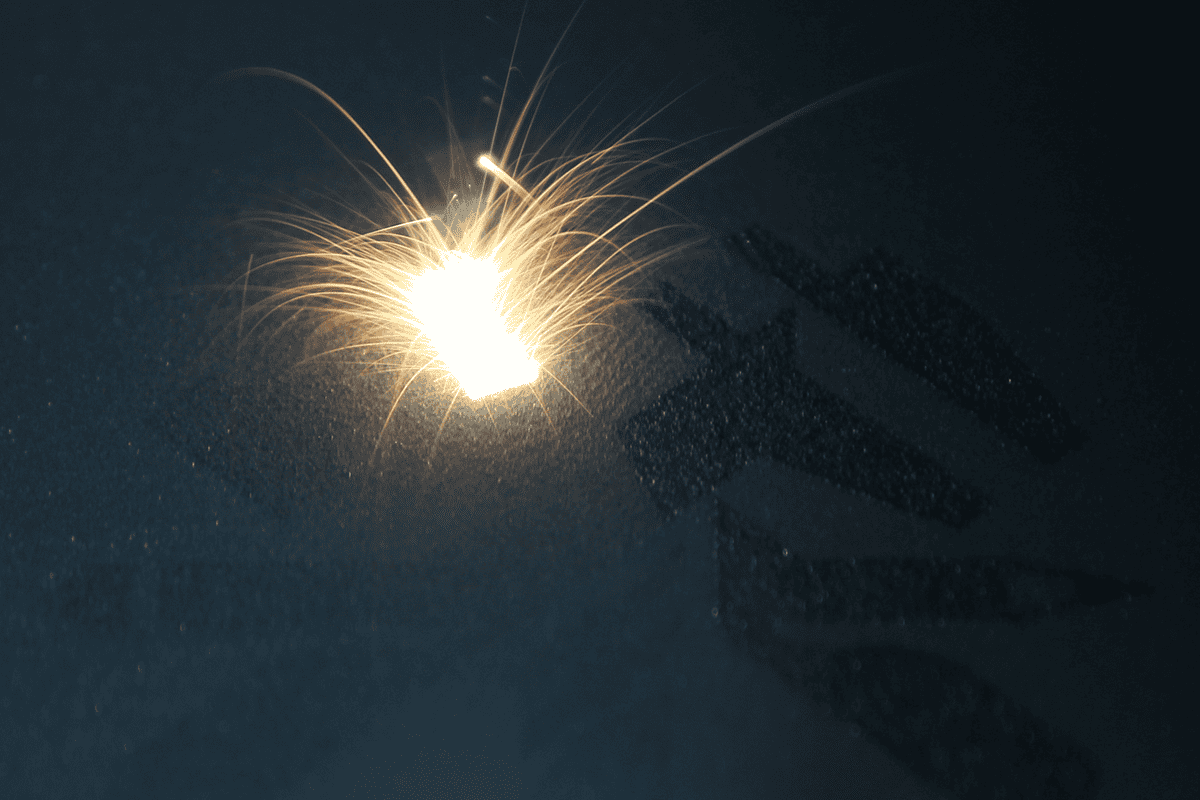
Direct Metal Laser Sintering (DMLS)
Are you developing a new product? Are you under pressure to bring it to market quickly? Do you need to validate the fit and finish of a metal part, but aren’t ready to have expensive tooling and fixtures made? DMLS can help you solve these problems and accelerate your time to market.
Additive Post-Processing Services
Our craftsmen and women go the extra mile to ensure that all your additive post-processing and finishing needs are met, quickly and efficiently, at the level of quality you expect.

Why Choose Fathom for 3D Printing?
We have over 40 years of expertise working with additive technologies. We also offer full post-processing capabilities under one roof.
When you contact Fathom to discuss your project, you’ll have immediate access to an engineer, who will help you select the right technologies, materials and processes that meet your requirements. Using our detailed DfAM process, we’ll help you optimize your part designs for the best results. We’ll work with you from prototype to production.
Our experts are ready to talk to you about your additive manufacturing project. Get started today with an online quote.
