- What is Additive Manufacturing?
- History of Additive Manufacturing
- How Does Additive Manufacturing Work?
- Advantages of Additive Manufacturing
- Additive Manufacturing Challenges
- Additive Manufacturing Materials & Processes
- How Long Does Additive Manufacturing Take?
- Top Industries Using Additive Manufacturing
- Should I Use Additive Manufacturing for My Project?
- Additive Manufacturing Questions Answered
- Additive Manufacturing Quote
Fathom’s industry-leading Additive Manufacturing (AM) and 3D printing services are ideal for accelerating product development and production processes. Fathom can accelerate your product development process from design to prototyping to manufacturing at a highly efficient and rapid pace. Companies from all industry categories have utilized Fathom’s team for low- to high-volume 3D printing and additive manufacturing. Our award-winning expertise, speed and quality make us one of the most trusted and innovative experts in the manufacturing industry.
Why Should You Work with Fathom on Your Additive Manufacturing Project?
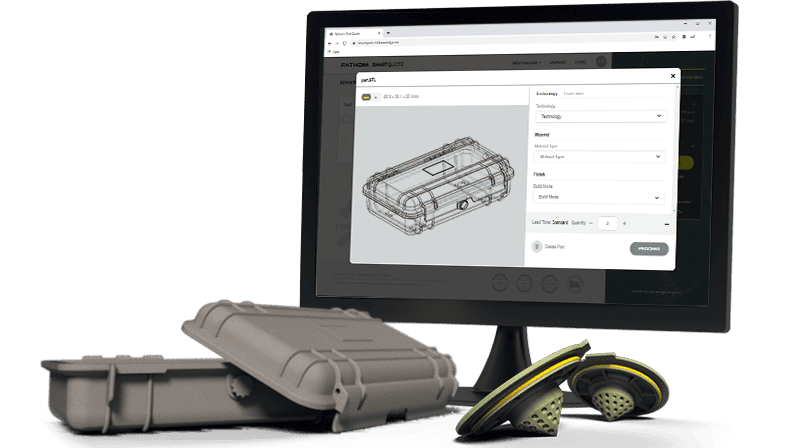
Using Fathom for your AM project is easy. Fathom utilizes, SmartQuote, a state-of-the-art on-demand online quoting platform that provides unlimited access to quotes and orders for prototyping and production parts. Our production team is ready to meet your needs with a wide range of processes and materials. Companies of all sizes trust Fathom from medical to consumer products, electronics, automotive, aerospace and more.
Receive instant quotes and quick turnaround times through Fathom’s SmartQuote platform.
Receive an Instant Quote for Fathom’s Additive Manufacturing Services
Using Fathom’s additive manufacturing quote platform, receive a rapid quote for your 3D printing or additive manufacturing job in just 3 easy steps.
- Upload Your CAD Design in the Proper Format
- Select Technology, Materials, Finishes & Quantity
- Review Your Quote & Make Any Modifications
To receive a complimentary quote, upload your CAD design file and provide us with details about your project. Fathom accepts uploads in the following formats (max files size up to 100 MB – utilize the manual quote form for larger files) //
| STL | OBJ | STEP | IGES | SLDPRT | 3DM | SAT | X_T |
For additional guidance on STL files, please refer to our STL guide.
Need additional help? We will connect you with an engineer who specializes in rapid manufacturing for complex projects. Fathom will help you choose technology and materials, improve functionality, drive part speed and more. Contact us today to get started.
How Quickly Will I Receive My 3D Quote and Additive Manufacturing Part? Most additive quotes are instant, but more complex files may need additional review. The lead time for parts depends on the complexity, quantity and technology used. Please refer to the chart below for approximate lead times.
| / / / / / / SAME-DAY | / / / / / / NEXT-DAY | / / / / / / TWO-DAY | / / / / / / THREE+ DAYS |
|---|---|---|---|
| PolyJet | PolyJet FDM SLA |
PolyJet FDM SLA SLS MJF |
PolyJet FDM SLA SLS MJF DMLS |
What is Additive Manufacturing?
Additive Manufacturing (AM) or additive layer manufacturing uses Computer-Aided Design (CAD) or a 3D model to direct machinery to build an object layer-by-layer. The CAD drawing acts as a set of instructions or blueprints, illustrating all the intricacies of the product being made. These blueprints instruct the machine where and when to place the material. Complex objects can be produced in this manner without the need to join together separate parts. This type of design freedom allows an engineer or industrial designer to improve functionality through increased complexity, which is often not the case with traditional manufacturing methods. The result is an intricate, unique and lightweight product with a high level of strength.
In this process, there are fewer errors as well as the opportunity to pinpoint and resolve problems before printing. To achieve a similar result using a traditional method, additional steps would be required such as carving, milling, machining or shaping. In other words, additive technologies enable the ability to fabricate geometries that were previously not manufacturable.
While additive technologies have mostly been used for prototyping, software and hardware materials have steadily matured to produce production-quality end-use parts—a process termed Direct Digital Manufacturing (DDM). This is driven by engineers and designers engaged in both day-to-day and big-picture design vision. Those with more knowledge and comfort with how to Design for Additive Manufacturing (DFAM) will be better able to leverage the value of this technology.
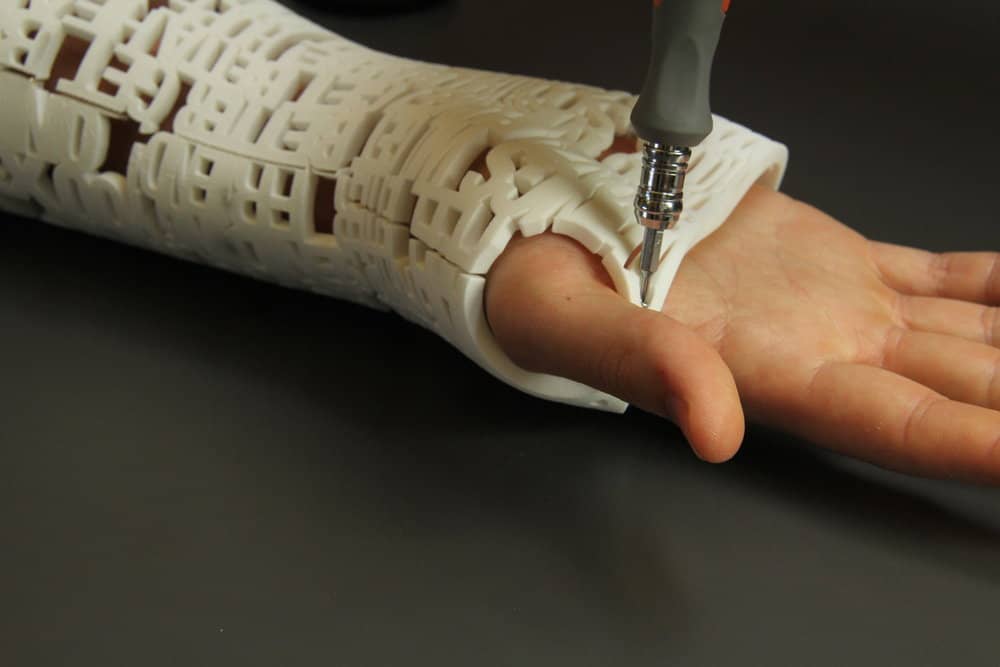
More than ever before, development can be front-loaded with a faster iteration of high-quality physical prototypes. Additive manufacturing can be applied to products from vastly different industries, including medical, dental, biotech, aerospace, automotive, construction, industrial design, education, fashion, food and more. Subsets of AM include 3D printing and metal 3D printing, Rapid Prototyping (RP), DFAM, layered manufacturing and additive fabrication.
History of Additive Manufacturing
While AM may seem like a new process, its origin goes back to the 1980s. Hideo Kodama of the Nagoya Municipal Industrial Research Institute was the first to publish an account of a functional rapid-prototyping system that used photopolymers. Using this RP system, a printed model was built in layers, each layer corresponding to a cross-sectional slice within the model. This was the birth of additive manufacturing. Since its invention, additive manufacturing has evolved rapidly and has found its use for thousands of applications.
How Does Additive Manufacturing Work?
The additive manufacturing process begins with design. First, a designer creates a blueprint using CAD or Computer-Aided Design software. Alternatively, a scan of an object may be taken. The software then builds the design layer-by-layer, making instructions for the additive manufacturing machine to follow. The design is then fed to the device and the product is produced. Additive manufacturing may use various materials including polymers, metals, glass, ceramics, foams, gels and even food and human organs.
Advantages of Additive Manufacturing
Additive manufacturing will provide your project with the freedom of a lightweight, inexpensive and strong product while still meeting the demands of an intricate design. Some of the additional advantages of additive manufacturing include //
- Allow for the creation of unique parts
- Enable clients to make rapid prototyping alterations during the production run to allow for a dynamic and design-driven manufacturing process
- Reduce lead times since a single component can be produced without the need for additional assembly steps
- Produce finished components through advancements in both methods and materials
- Produce less waste than traditional subtractive processes
- Allow for low-volume production runs reducing time and money investments
- Provide limitless applications that are appropriate for numerous industries
Additive Manufacturing Challenges
While additive manufacturing has many advantages, there are some disadvantages as well. These include //
- Machines may cost hundreds of thousands of dollars if you choose to do additive manufacturing yourself
- Objects produced by additive manufacturing typically require some post-process cleaning or smoothing
- New materials are constantly being developed & technology processes continue to be refined
Additive Manufacturing Technologies Overview
There are three broad categories of additive manufacturing.
Sintering // Material is heated but not liquified, which results in a complex and high-resolution object. Direct Metal Laser Sintering uses metal powder. Selective Laser Sintering uses lasers on thermoplastic powders, which causes the particles to stick together.
Melting // The material is fully melted. This technology includes DMLM which directs a laser to melt layers of metal powder and electron beam melting that uses an electron beam to melt the powder.
Stereolithography // Using photopolymerization, ultraviolet lasers are fired into photopolymer resin. The result is torque-resistant ceramic parts that can withstand extreme temperatures.
Fathom Additive Manufacturing Materials and Processes
PolyJet (PJ)
PolyJet technology can create smooth surfaces, thin walls and complex geometries with accuracy as high as 0.1 mm—the only technology that supports a wide selection of materials with properties that range from rubber to rigid and transparent to opaque. It is also possible to 3D print with multiple materials in a single build to achieve combinations of colors and characteristics (e.g., parts made of rigid and flexible materials).
How does the PJ technology work? PJ is a photopolymer-based jetting process that distributes material droplets layer-by-layer onto a build platform (immediately cured by a flash of UV light). At the end of the build process, the object is fully cured and can be handled immediately without post-curing. This technology includes using a gel-like support material, designed to enable complicated geometries (removed by soaking and/or water jetting). Learn more about PolyJet.
PolyJet Parts In As Soon As Same-Day //Get A Quote